XRD and XRF: aiding the enhancement of cementitious materials – Part 2
Read Part 1 here.
Cement pathologies
The behaviour of cement in different environments can also be measured by using XRD and XRF. It is possible to recognise the hydrated phase’s degradation through the use of acid solutions, or the formation of new phases in different atmospheres or solutions. In a CO2 atmosphere, for example, it is possible to find CaCO3 in an aragonite and/or calcite form, which is produced as a result of the reaction between the free calcium hydroxide and the CO2 gas. Also, in sulfate solutions, it is possible to follow ettringite increases and thaumasite formation, which are phases that are produced as a result of more complex reactions between hydrated calcium aluminates and hydrated calcium silicate and alkaline sulfates (such as Na2SO4 and K2SO4) that come from the sulfate solution.
The effects of other compounds
Other materials and phases that are very common and known in cement analysis are calcium sulfate (gypsum) and C4AF (Brownmillerite). Nevertheless, research has focused more on other phases of clinker, such as C3S, C3A and C2S, and other materials, such as SCMs, limestone, chemical additives, etc. There has not been much research about the two formerly mentioned and important compounds, which will always be present in cement and which, in some ways, affect the main reaction in the hydration process of Portland cement.
In the case of gypsum (CaSO4•2H2O), it is clear that its role is to control the setting time of cement when it reacts with C3A, delaying the hydration and giving end users time to handle and pour the product. This is a very useful characteristic as accelerating or delaying the setting time is a common request in the industry. On the other hand, the amount of gypsum has to be optimal in the formulation of cement because an excessive or low dosage will have an effect on the setting time and it will also affect the durability. Furthermore, gypsum is an expensive material. All of these aspects can be controlled through the correct identification of the main phases of calcium sulfate (Figure 3), dihydrate or gypsum, hemihydrate or bassanite and anhydrite, because the behaviour of the mineral is not only affected by its purity – usually measured as the amount of SO3 – but also by the relative presence of these phases, due to their different capabilities in controlling the hydration of the cement.
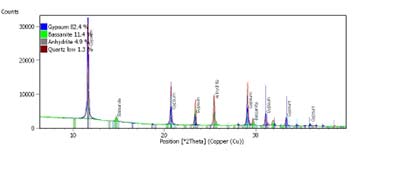
Figure 3. XRD analysis of a calcium sulfate source. (Source: Argos Research and Development Center.)
In the case of C4AF (Figure 4), in the formation of clinker it acts as a fluxing agent and allows the formation of the main phases through the ion interchange between the chemical species. However, what are the effects of increasing the amount of iron in the raw materials? The first result is that higher amounts of C4AF will be detected in the clinker. So, a new question arises: what happens during the cement hydration process and to the final performance of cement when there are high amounts of C4AF? The answer is that C4AF stimulates the formation of a layer that delays the setting time and the normal development of strength, not only as a consequence of less C3S formation, but also because this phase has its own pattern and is needed during hydration.
Again, a question arises: how can this particular situation be identified? This can be achieved via a complete analysis of the hydration of pure clinker phases using XRF and XRD tools.
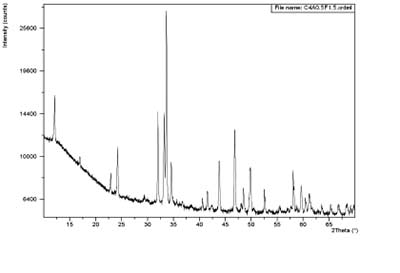
Figure 4. XRD analysis of iron rich calcium aluminate (C4A0.5F1.5). (Source: Argos Research and Development Center.)
New functionalities
With the increasing need for new houses, buildings, roads, etc., new materials and more specific applications and functionalities are demanded of construction materials. More environmentally friendly and intelligent materials have to be developed for the future. An environmentally friendly material can mean, for example, a material that allows us to reduce energy consumption in houses and buildings. This is a challenge for materials science because scientists need to think about materials that keep their temperature in indoor spaces. Perhaps they need to devise materials that allow light to pass through (translucent or transparent materials), or maybe they need to think of cement materials that can conduct electricity. Other opportunities could be found in the area of cleaner materials, such as photocatalytic types of cement that absorb NOX and SOX and that could thus allow us to develop a material that absorbs other contaminants from the air, water or soil. Finally, there are also the so-called intelligent materials, which can provide useful information about the state of the structure after many years of use or, for example, after an earthquake or similar situation. No doubt the development of these materials will need the help of advances in technical analysis and tools such as XRF and XRD.
Conclusion
Technical analysis tools such as XRD and XRF represent a significant help to R&D teams in the cement industry when it comes to understanding and developing new products. The complete chemical and mineral characterisation of the materials used in cement production and the products generated after the hydration process play an important role in the process of obtaining more detailed and accurate knowledge of all the mechanisms and phenomena that govern the reactions and functionalities involved in its production and performance. More refined technical analysis tools have to be developed in order to be able to gain a deeper insight into the structures and to identify all the aspects of the relationship between molecules and atoms and, in some way, manipulate them in order to produce the materials that we need to transform our environment whilst remaining in harmony with nature.
Written by Juan Guillermo Morales, Claudia Rodriguez, Carolina Giraldo and Ruby Estela Cardona, Research and Development Department, Cementos Argos S.A.
Bibliography
- TAYLOR, H.F.W, Chemistry of Cement, 2nd Edition (1997).
- KOMNITSAS, K., and ZAHARAKI, D., ‘Geopolymerisation: A review and prospects for the minerals industry’, Minerals Engineering20 (2007) pp. 1261 – 1277.
- THOMAS, M.D.A, and HOOTON, R.D., ‘The Durability of Concrete Produced with Portland-Limestone Cement: Canadian Studies’, Portland Cement Association (2010).
- BENTZ, D.P., et al., ‘Fine limestone additions to regulate setting in high volume flyash mixtures’, Cement & Concrete Composites 34 (2012), pp. 11 – 17.
- LOTHENBACH, B., et al., ‘Influence of limestone on the hydration of Portland cements’, Cement and Concrete Research 38 (2008), pp. 848 – 860.
- SCRIVENER, K.L., and KIRKPATRICK, R.J., ‘Innovation in use and research on cementitious material’, Cement and Concrete Research 38 (2008), pp. 128 – 136.
- World Cement, ‘XFR and XRD based solutions’, February 2004.
- WESSELSKY, A., and JENSEN, O.M., ‚Synthesis of pure Portland cement phases’, Cement and Concrete Research 39 (2009), pp. 973 – 980.
Read the article online at: https://www.worldcement.com/the-americas/14012014/xrd_and_xrf_analysis_cementos_argos_part_2_593/
You might also like
The World Cement Podcast - CleanTech & Venture Capital
Our guest for this episode of the World Cement Podcast is Alfredo Carrato, Venture Capital Advisor for CEMEX Ventures. Listen in to the conversation as World Cement's Senior Editor, David Bizley, and Alfredo discuss the role of venture capital and cleantech in enabling the cement industry's green transition.
Tune in to the World Cement Podcast on your favourite podcast app today.


